“Turning water into wine” is the first miracle attributed to Jesus in the Gospel of John, as depicted in one of the renowned painings by Paolo Veronese, in Musée du Louvre in Paris. “Is it scientifically possible ?”, asked many inquisitive minds, not necessarily to meet the craving for drinking but to find a “carbon-neutral” fuel for driving.

(The Wedding Feast at Cana, 1563, Paolo Veronese, Musée du Louvre; Courtesy Wikipedia)
At least, in a new article in Nature Communications (see link at the end), an international team of collaborators from UCL, Imperial College London, St Andrews, The University of British Columbia and HKU / HKU-ZIRI have scientifically demonstrated the possibility of transforming “water and, even better, carbon dioxide”, into methanol alcohol (OK – definitely not for drinking!) under solar irridiation, with the assistance of a type of tiny carbon-dot on semi-conductive carbon-nitride, as a highly selective photocatalyst.
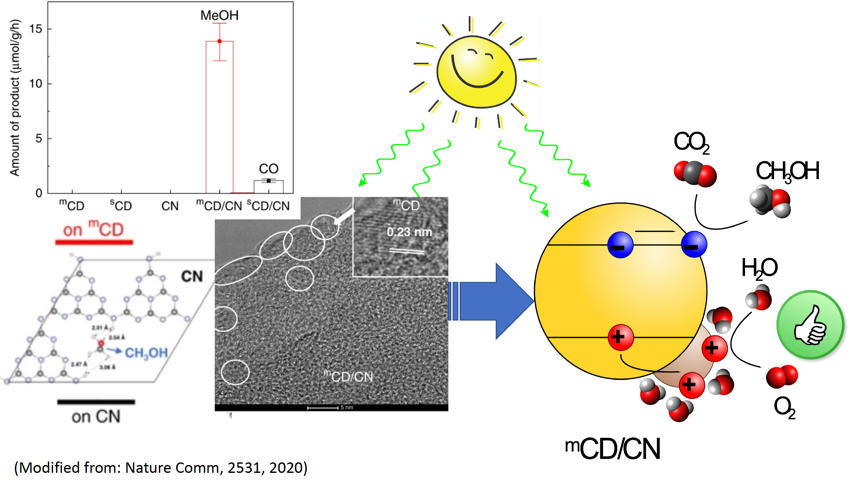
In fact, methanol is both a valuable chemical and an effective liquid fuel alternative to petrol. With the team’s effort, it can now be sustainably produced via a carbon-neutral pathway using only sunlight, greenhouse gas CO2 and water with unity selectivity! By careful design and integration of carbon-dots/carbon-nitride (CD/CN) junction, the authors show stable conversion of water and CO2 to stoichiometric O2 and methanol with nearly 100% selectivity under sunlight. The key behind these new exciting results is the unique and efficient hole-accepting nature of the highly graphitic CD and its selective adsorption of water over methanol。
Two photoreactions are driven by the system: 1) water (H2O) oxidation and 2) CO2 reduction to high value products, in this case methanol (CH3OH). High performance photocatalysts utilize co-catalysts to enhance the catalytic properties of the surface and accelerate the rate of reaction. The heterojunction between the light-absorbing photocatalyst and the co-catalyst also makes electrons and holes flow to different materials and limit how fast they find each other (leading to the annihilation reaction termed recombination). This translates to an increase in lifetime and a greater chance they do the desired reaction.
Computational results showed that water prefers adsorption to mCD (where holes accumulate) while methanol prefers adsorption to CN (where electrons accumulate), thus facilitating selective oxidation of water to O2 while avoiding unproductive oxidation of the photo-reduced product, methanol. The production of methanol proceeds with an exceptional near-unity selectivity and a high IQY of 2.1% at 420 nm (with benchmark IQYs of 0.7% and 0.4% at 500 and 600 nm, respectively).
This strategy selectively reduces greenhouse gas by water to high-value chemicals, hence offering sustainable “carbon-zero” liquid fuels and closing the global carbon cycle. For more details on this study, please see our recent article [link below] “Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction 100% to methanol by pure water” in Nature Communications.
Link:https://www.nature.com/articles/s41467-020-16227-3