“化水为酒”据说是耶稣创造的第一个奇迹,(见图:保罗·韦罗内塞的著名画作;存于巴黎卢浮宫)。也许有史以来,许多人会好奇的问“化水为酒,这在科学上是有可能的吗?”- 当然,不一定是想喝酒,而是寻找一种可以缓解气候变化、“零碳排放”的新能源。
“Turning water into wine” is the first miracle attributed to Jesus in the Gospel of John, as depicted in one of the renowned painings by Paolo Veronese, in Musée du Louvre in Paris. “Is it scientifically possible ?”, asked many inquisitive minds, not necessarily to meet the craving for drinking but to find a “carbon-neutral” fuel for driving.

(The Wedding Feast at Cana, 1563, Paolo Veronese, Musée du Louvre; Courtesy Wikipedia)
近期,在《自然.通讯》的一篇新文章中[见文末链接],来自伦敦大学学院、伦敦帝国理工学院、圣安德鲁斯大学、英属哥伦比亚大学和香港大学/香港大学浙江科学技术研究院的国际合作团队证明了在太阳辐射下,借助于“微波碳点”的光空穴引导作用使氮化碳成为高选择性的光催化剂,可将“水,加上二氧化碳”转化为甲醇(绝对不能喝!)。
At least, in a new article in Nature Communications (see link at the end), an international team of collaborators from UCL, Imperial College London, St Andrews, The University of British Columbia and HKU / HKU-ZIRI have scientifically demonstrated the possibility of transforming “water and, even better, carbon dioxide”, into methanol alcohol (OK – definitely not for drinking!) under solar irridiation, with the assistance of a type of tiny carbon-dot on semi-conductive carbon-nitride, as a highly selective photocatalyst.
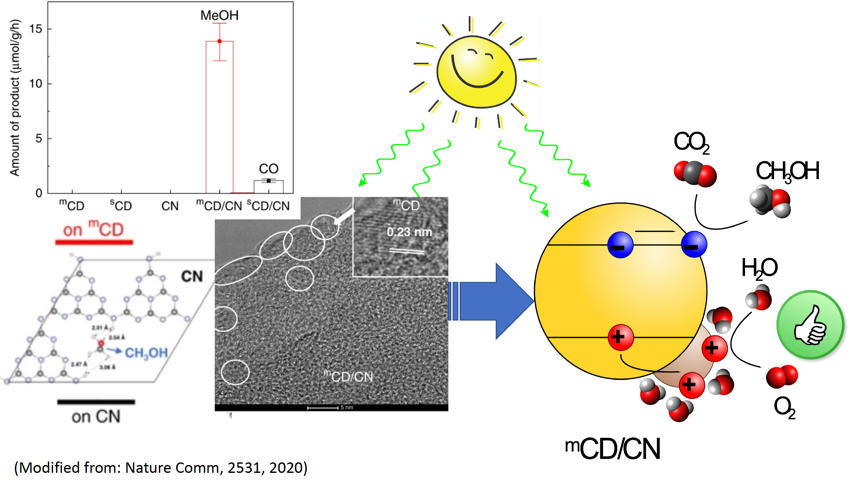
事实上,甲醇既是一种有价值的化学产品,也是一种有效的清洁液体燃料,可用于燃料电池或内燃机。团队的研究成果表明,甲醇可通过“零碳排放”的方法通过太阳光将水和温室气体-二氧化碳直接转化。通过对碳点/氮化碳(CD/CN)复合材料的设计和嵌合,团队证明了水和二氧化碳在阳光下以接近100%选择性,稳定地将二氧化碳同比转化为氧气和甲醇。这项激动人心的新成果背后的关键是在于,高石墨化碳点独特而高效的空穴接受特性及其对水的选择性吸附能力。
In fact, methanol is both a valuable chemical and an effective liquid fuel alternative to petrol. With the team’s effort, it can now be sustainably produced via a carbon-neutral pathway using only sunlight, greenhouse gas CO2 and water with unity selectivity! By careful design and integration of carbon-dots/carbon-nitride (CD/CN) junction, the authors show stable conversion of water and CO2 to stoichiometric O2 and methanol with nearly 100% selectivity under sunlight. The key behind these new exciting results is the unique and efficient hole-accepting nature of the highly graphitic CD and its selective adsorption of water over methanol。
该系统主要包含两种光化学反应:1)水(H2O)的氧化和2)二氧化碳(CO2)还原成高价值产物-甲醇(CH3OH)。在这里,通过利用协同催化效应,提高材料表面的催化性能,加快反应速度;吸收光子的光催化剂和协同催化剂之间的异质结也使电子和空穴流向不同的材料表面,并限制它们的相互重组(湮灭)反应。这样的反应设计使光电子和空穴有更长的生命周期,进而有更多的机会去完成有效的光化学反应。
Two photoreactions are driven by the system: 1) water (H2O) oxidation and 2) CO2 reduction to high value products, in this case methanol (CH3OH). High performance photocatalysts utilize co-catalysts to enhance the catalytic properties of the surface and accelerate the rate of reaction. The heterojunction between the light-absorbing photocatalyst and the co-catalyst also makes electrons and holes flow to different materials and limit how fast they find each other (leading to the annihilation reaction termed recombination). This translates to an increase in lifetime and a greater chance they do the desired reaction.
理论模拟结果表明,mCD(空穴聚集处)对水有更强的吸附作用,同时,CN对甲醇有着更强的选择吸附作用(电子聚集导致),因此,mCD/CN系统促进了水到氧气的选择性氧化,同时有效的避免了对光还原产物甲醇的氧化。该系统催化生产的甲醇具有近于100%的选择性和较高内量子效率(2.1%在420nm,相比于在500 nm和600 nm处的基准IQY分别为0.7%和0.4%)。(IQY:internal quantum yield 内量子效率)
Computational results showed that water prefers adsorption to mCD (where holes accumulate) while methanol prefers adsorption to CN (where electrons accumulate), thus facilitating selective oxidation of water to O2 while avoiding unproductive oxidation of the photo-reduced product, methanol. The production of methanol proceeds with an exceptional near-unity selectivity and a high IQY of 2.1% at 420 nm (with benchmark IQYs of 0.7% and 0.4% at 500 and 600 nm, respectively).
这一方法可以有选择地讲温室气体转化为高价值的化学产品,从而可持续的提供“零碳”液体燃料,并实现整体的温室碳循环。关于这项研究的更多细节,请参阅团队最近在《自然.通讯》上发表的文章[见文末]“独特的空穴接受碳点促进纯水(吸附氧化)使二氧化碳100%还原为甲醇”。
This strategy selectively reduces greenhouse gas by water to high-value chemicals, hence offering sustainable “carbon-zero” liquid fuels and closing the global carbon cycle. For more details on this study, please see our recent article [link below] “Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction 100% to methanol by pure water” in Nature Communications.
原文链接:https://www.nature.com/articles/s41467-020-16227-3